Cannabidiol In Patients With Treatment-resistant Epilepsy: An Open-label Interventional Trial
Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. 13 In two 14-week randomized double-blind placebo-controlled studies that enrolled patients with LGS GWPCARE3 and GWPCARE4 add-on CBD resulted in a reduced frequency of drop seizures vs placebo improved patient. Cannabidiol in patients with treatment-resistant epilepsy. The Lancet Neurology.
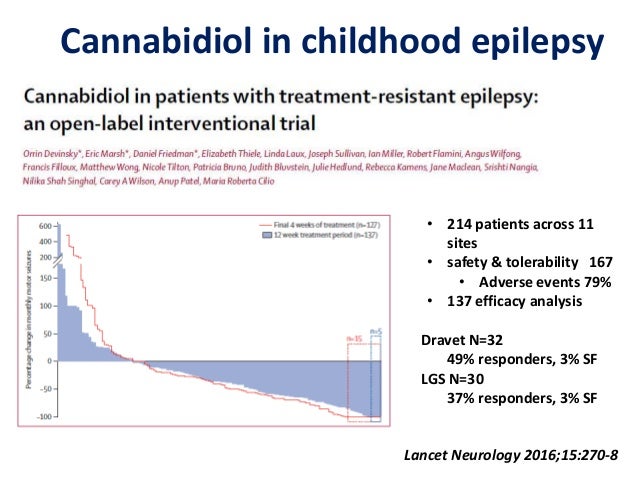
Devinsky O Marsh E Friedman D et al. Our findings suggest that cannabidiol might reduce seizure frequency and might have an adequate safety profile in children and young adults with highly treatment-resistant epilepsy. 27078The appendix of this Article has been resupplied to include the correct number of patients at each study site in supplementary table 1.
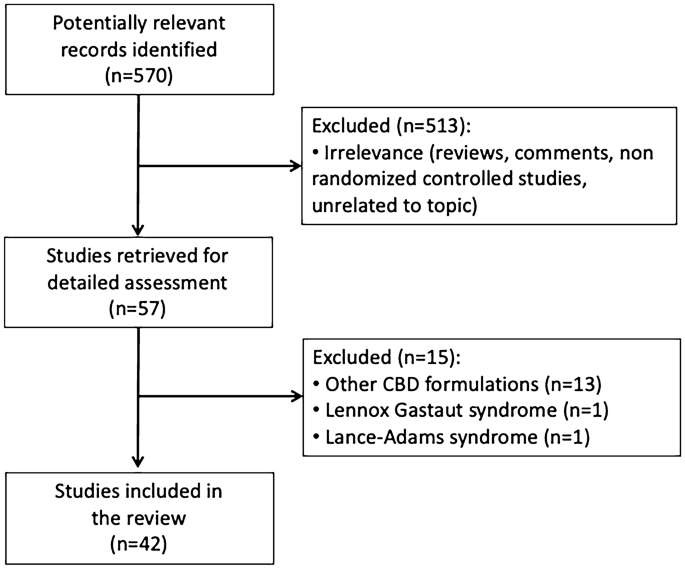
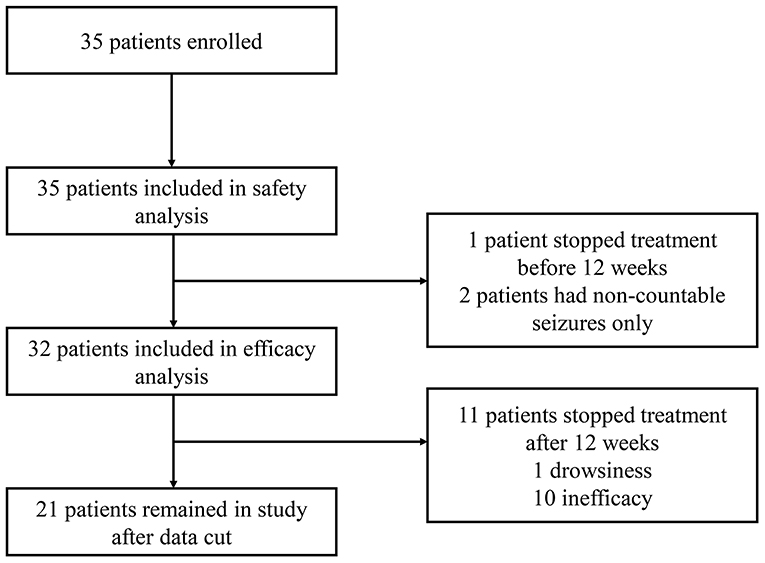
Methods In this open-label trial patients aged 130 years with severe intractable childhood-onset treatment-resistant epilepsy who were receiving stable doses of antiepileptic drugs before study entry were enrolled in an expanded-access programme at 11 epilepsy centres across the USA. This correction has been made to the online version as of March 7 2016. An open-label interventional trial.
Zolgensma Benefits Observed in Presymptomatic SMA Patients March 28 2021 News ICNA According to research presented at the 2021 Muscular Dystrophy Association MDA Virtual Clinical and Scientific Conference on the SPR1NT trial Children with SMA treated with onasemnogene abeparvovec-xioi Zolgensma. An open-label interventional trial. An open-label interventional trial.
Cannabidiol in patients with treatment-resistant epilepsy. Almost a third of patients with epilepsy have a treatment-resistant form which is associated with severe morbidity and increased mortality. The long-term efficacy and safety of cannabidiol is currently being assessed in the open-label extension of this trial.
Patients were given oral cannabidiol at 2-5 mgkg per day up-titrated until intolerance or to a maximum dose of 25 mgkg. An open-label interventional trial. This trial shows that long-term CBD treatment had an acceptable safety profile and led to sustained clinically meaningful reductions in seizure frequency in patients with treatment-resistant DS.
An open-label extension trial. In this open-label trial patients aged 130 years with severe intractable childhood-onset treatment-resistant epilepsy who were receiving stable doses of antiepileptic drugs before study entry were enrolled in an expanded-access programme at 11 epilepsy centres across the USA.
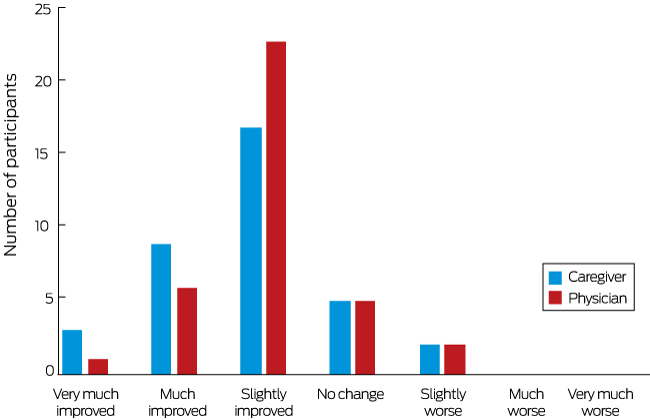
Reductions in convulsive and total seizure frequency observed in the original placebo-controlled trial were maintained with continued CBD treatment up to 48 weeks with more than 80 of patientscaregivers reporting an improvement in.
In this open-label trial patients aged 130 years with severe intractable childhood-onset treatment-resistant epilepsy who were receiving stable doses of antiepileptic drugs before study entry were enrolled in an expanded-access programme at 11 epilepsy centres across the USA. An open-label interventional trial. In our open-label study add-on treatment with pure cannabidiol led to a clinically meaningful reduction in seizure frequency in many patients and had an adequate safety profile in this patient population with highly treatment-resistant epilepsies. Academic Article Overview abstract. The Lancet Neurology. Long-term cannabidiol treatment in patients with Dravet syndrome. Cannabidiol in patients with treatment-resistant epilepsy. Devinsky O Marsh E Friedman D et al. In this open-label trial patients aged 130 years with severe intractable childhood-onset treatment-resistant epilepsy who were receiving stable doses of antiepileptic drugs before study entry were enrolled in an expanded-access programme at 11 epilepsy centres across the USA.
Reductions in convulsive and total seizure frequency observed in the original placebo-controlled trial were maintained with continued CBD treatment up to 48 weeks with more than 80 of patientscaregivers reporting an improvement in. In this open-label trial patients aged 1-30 years with severe intractable childhood-onset treatment-resistant epilepsy who were receiving stable doses of antiepileptic drugs before study entry were enrolled in an expanded-access programme at 11 epilepsy centres across the USA. Long-term cannabidiol treatment in patients with Dravet syndrome. Cannabidiol in patients with treatment-resistant epilepsy. Patients were given oral cannabidiol at 25 mgkg. An open-label interventional trial Author Creator Devinsky Orrin Dr Prof Marsh Eric MD Friedman Daniel MD Thiele Elizabeth Prof Laux Linda MD Sullivan Joseph MD. An open-label extension trial.



































Post a Comment for "Cannabidiol In Patients With Treatment-resistant Epilepsy: An Open-label Interventional Trial"